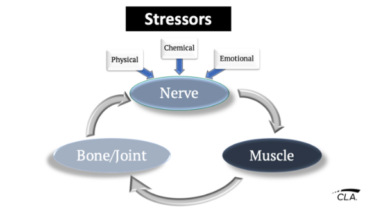
Subluxation degeneration has been described as a progressive process associated with abnormal spinal mechanics. The degenerative changes are associated with various mechanisms of neurological dysfunction. (1)
Progressive degeneration of the cervical spine is thought to begin with the intervertebral discs, progressing to changes in the cervical vertebrae and contiguous soft tissues. (2)
Several early investigators explored the relationship of spinal degenerative disease to neurological compromise.
In 1838, Key described a case of cord pressure due to degenerative changes causing spinal canal stenosis. (3)
Bailey and Casamajor reported that cord compression could result from spinal osteoarthritis. They suggested that disc thinning was the basic pathology underlying degenerative change. (4)
As early as 1926, Elliott gave an account of how radicular symptoms could be caused by foraminal stenosis secondary to arthritic changes. (5)
Several mechanisms have been suggested which may be operative in cervical spine degeneration.
Resnick and Niwayama used the term “intervertebral (osteo)chondrosis” to describe abnormalities which predominate in the nucleus pulposus. (6)
Osteoarthritis of the uncovertebral and zygapophyseal joints is another manifestation of cervical spine degeneration. Spondylosis is the term these authors applied to degenerative changes which occur as a result of enlarging annular defects which lead to disruption of the attachment sites of the disc to the vertebral body. This leads to the appearance of osteophytes.
O’Connell employed the term “spondylosis” in a broader context. Three lesions were described: disc protrusion into the intervertebral canal; primary spondylosis, characterized by degenerative changes between the vertebral bodies and zygapophyseal joints; and secondary spondylosis, associated with disc protrusion at a single spinal level. (7)
In the lumbar spine, pathomechanics and torsional stress have been implicated as etiological factors in spinal degeneration. (8,9)
It is likely that these factors are operative in the pathogenesis of cervical spine degeneration as well. Although it has been suggested that aging is responsible for degenerative changes in the spine, this appears to be an oversimplification. (10)
For example, Lestini and Weisel report that there is a high statistical correlation between disc degeneration and posterior osteophyte formation. (2)
Furthermore, it is noted that the incidence of degenerative changes varies from one segmental level to another. The C5/C6 level is most frequently involved, with C6/C7 being the level next most frequently affected. The C2/C3 level is the one least likely to exhibit degenerative changes. (11)
Since the prevalence of cervical spine degenerative change is not uniform throughout the region, the hypothesis that degenerative change is associated with spinal pathomechanics deserves consideration.
Hadley suggests that both aging and pathomechanics are operative in the pathogenesis of cervical spine degeneration. Age related disc degeneration causes hypermobilty, resulting in greater tractional forces on ligaments. This is said to result in the formation of reactive osteophytes. Trauma can result in local spondylotic changes. (11)
This is similar to MacNab’s description of traction spur formation in the lumbar spine. (12)
Pesch et al measured the dimensions of the fifth, sixth, and seventh cervical vertebral bodies in 105 cadavers aged 16 to 91 years. Similar measurements were made on the third, fourth, and fifth lumbar vertebral bodies.
The authors suggest that dynamic stressing of the cervical vertebral bodies leads laterally to friction between vertebral bodies at the uncovertebral joints, causing osteophytosis. Anteriorly, osteophytic formation is attributed weakness of the anterior longitudinal ligament, leading to anterior disc protrusion. (13)
Neurological consequences of spinal degeneration
Neurological manifestations of spinal degeneration may be due to a variety of mechanisms. These include:
1. Cord compression. Compression of the spinal cord may result from disc protrusion, ligamentum flavum hypertrophy/corrugation, or osteophytosis. Myelopathy may result in cord pressure and/or pressure which interferes with the arterial supply. (7,14,15,16)
Payne and Spillane found that myelopathy was more likely to occur in persons with congenitally small spinal canals who subsequently develop spondylosis. (17)
Hayashi et al report that in the cervical region, dynamic canal stenosis occurs most commonly in the upper disc levels of C3/C4 and C4/C5. (18)
2. Nerve root compression. Compromise of the nerve roots may develop following disc protrusion or osteophytosis. Symptoms are related to the nerve root(s) involved. (19)
3. Local irritation. This includes irritation of mechanoreceptive and nociceptive fibers within the intervertebral motion segments. MacNab reports that arm pain may occur without evidence of root compression. The pain is attributed to cervical disc degeneration associated with segmental instability. (19)
4. Vertebral artery compromise. MacNab advises that osteophytes may cause vertebral artery compression. (19)
Furthermore, Smirnov studied 145 patients with pathology of the cervical spine and cerebral symptoms. 59% had vertebrobasilar circulatory disorders. (20)
5. Autonomic dysfunction. Symptoms associated with the autonomic nervous system have been reported. The Barre’-Lieou syndrome includes blurred vision, tinnitus, vertigo, temporary deafness, and shoulder pain. This phenomenon occurs following some cervical injuries, and is also known as the posterior cervical syndrome. (21)
Stimulation of sympathetic nerves has been implicated in the pathogenesis of this syndrome. (22)
Another manifestation of autonomic involvement, reflex sympathetic dystrophy, results in shoulder and arm pain accompanied by trophic changes. (23)
References
1. Flesia J: “Renaissance — A Psychoepistemological Basis for the New Renaissance Intellectual.” Renaissance International, Colorado Springs, CO, 1982.
2. Lestini WF, Wiesel SW: “The pathogenesis of cervical spondylosis.” Clin Orthop (1989 Feb) 238:69.
3. Key CA: “On paraplegia depending on the ligaments of the spine.” Guy’s Hosp Rep (1838) 3:17.
4. Bailey P, Casamajor L: “Osteoarthritis of the spine as a cause of compression of the spinal cord and its roots.” J Nerv Ment Dis (1911) 38:588.
5. Elliott GR: “A contribution to spinal osteoarthritis involving the cervical region.” J Bone Joint Surg (1926) 8:42.
6. Resnick D, Niwayama G: “Diagnosis of Bone and Joint Disorders, Volume 3.” WB Saunders Co., Philadelphia, PA, 1988.
7. O’Connell JE: “Involvement of the spinal cord by intervertebral disc protrusions.” Br J Surg (1955) 43:225.
8. Miller J, Schmatz B, Schultz A: “Lumbar disc degeneration: Correlation with age, sex, and spine level in 600 autopsy specimens.” Spine (1988) 13:173.
9. Farfan HF, Cossette JW, Robertson GH, Wells RV: “The effects of torsion on the lumbar intervertebral joints: The role of torsion in the production of disc degeneration.” J Bone Joint Surg (Am) (1970) 52A(3):468.
10. Kent C, Holt F, Gentempo P: “Subluxation degeneration in the lumbar spine: Plain film and MR imaging considerations.” ICA Review (Jan/Feb 1991) 47(1):55.
11. Hadley LA: “Anatomico-Roentgenographic Studies of the Spine.” Charles C. Thomas, Springfield, IL. 1981. Chapters IV and IX.
12. MacNab I: “The traction spur: An indicator of segmental instability.” J Bone Joint Surg (1971) 53A:663.
13. Pesch HJ, Bischoff W, Becker T, Seibold H: “On the pathogenesis of spondylosis deformans and arthrosis uncovertebralis: comparative form- analytical radiological and statistical studies on lumbar and cervical vertebral bodies.” Arch Orthop Trauma Sur (1984) 103(3):201.
14. Taylor AR: “Mechanism and treatment of spinal cord disorders associated with cervical spondylosis.” Lancet (1953) 1:717.
15. Mair WG, Druckman R: “The pathology of spinal cord lesions and their relations to the clinical features in protrusion of cervical intervertebral discs.” Brain (1953) 76:70.
16. Maiuri F, Gangemi M, Gambardella A, Simari R, D’Andrea F: “Hypertrophy of the ligamenta flava of the cervical spine. Clinico- radiological correlations.” J Neurosurg Sci (1985) 29(2):89.
17. Payne EE, Spillane JD: “The cervical spine. An anatomico- pathological study of 70 specimens (using a special technique) with particular reference to the problem of cervical spondylosis.” Brain (1957) 80:571.
18. Hayashi H, Okada K, Ueno R: “Etiologic factors of cervical spondylitic myelopathy in aged patients — clinical and radiological studies.” Nippon Seikeigeka Gakkai Zasshi (1987) 61(10):1015. (Published in Japanese–English abstract).
19. MacNab I: “Cervical spondylosis.” Clin Orthop (1975) 109:69.
20. Smirnov VA: “The clinical picture and pathogenesis of cerebral symptomatology in diseases of the cervical region of the spine.” Zh Nervopatol Psikhiatr (1976) 76(4):523. Published in Russian — English abstract).
21. Barre’ JA: “Sur un syndrome sympathique cervical posterieur et sa cause frequente, 1, artrite cervicale.” Rev Neurol (Paris) (1926) 1:1246. Published in French.
22. Watanuki A: “The effect of the sympathetic nervous system on cervical spondylosis.” Nippon Seikeigeka Gakkai Zasshi (1981) 55(4):371. Author’s translation.
23. Wainapel SF: “Reflex sympathetic dystrophy following traumatic myelopathy. Pain (1984) 18:345.